Common or well known photochemical oxidants include ozone (O3), hydrogen peroxide (H2O2), and peroxyacetyle nitrate (PAN). Examples of photochemical oxidants are: Ozone. Peroxyacetyle nitrate. The yield and quality of flowers, fruits, cones, and seeds often are decreased by exposure to pollutants in the air and soil.
Smog is air pollution consisting of smoke and fog. Many solvents undergo chemical reactions in the atmosphere, leading to a number of indirect effects, including the formation of photochemical oxidants and ozone. While air pollution from coal no longer plagues urban areas, that from diesel vehicles and other aspects of modern life, including precursor chemicals,. These precursor compounds include the oxides of nitrogen and a variety of hydrocarbons with different chemical reactivities with respect to the formation of photochemical oxidants. Ozone and other photochemical oxidants (such as peroxyacyl nitrates and aldehydes) are formed by the action of ultra-violet (UV) light from the sun on nitrogen oxides (a process called photolysis ). Its production and concentration is dependent on the presence of primary pollutants as well as ultra-violet light.
The most adverse components formed by photochemical reactions in polluted air are ozone (). Ground-level ozone (O 3) is not emitted directly from anthropogenic sources. It is a “secondary” pollutant formed by a complicated series of chemical reactions in the presence of sunlight.
Under typical daytime conditions with a well-mixed atmosphere, three reactions reach equilibrium and no net chemistry occurs. Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible light or infrared radiation.
Photochemical reactions proceed differently than temperature-driven reactions. The most important phytotoxic components produced by these atmospheric photochemical reactions are ozone and peroxyacetyl nitrate. National Research Council. The photochemical oxidants are secondary air pollutants formed by the action of sunlight on nitrogen oxides and reactive hydrocarbons, their precursors.
These laws led to a rapid decrease in the concentration of photochemical oxidants , resulting in a sharp decrease in the number of reports of individuals harmed by photochemical oxidants. However, in recent years, photochemical oxidant concentrations throughout Japan have, once again, begun to increase. Even small traces of these chemicals can affect the respiratory tract of humans and animals, and damage crops and trees. Nitric oxide combines with water vapour in the atmosphere to form nitric aci which is one of the components of acid rain. Heightened levels of atmospheric nitric oxide resulting from industrial activity were also one of the causes of gradual depletion of the….
An uncorrected copy, or prepublication, is an uncorrected proof of the book. The final version of this book has not been published yet. You can pre-order a copy of the book and we will send it to you when it becomes available. The term oxidant is often used loosely and deserves clarification. Very often, it refers implicitly to 0 the most abundant oxidant in the troposphere.
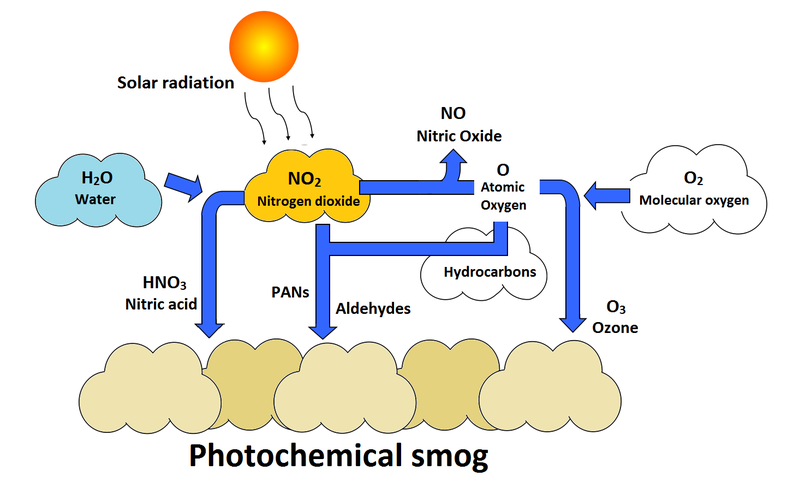
Starch-filled leaves of plants which have been subjected to low dosages of naturally occurring photochemical oxidants , ozone, or peroxyacetyl nitrate hydrolyze their starch more slowly when placed in the dark. Delayed hydrolysis occurs irrespective of whether the oxidants were applied during the light or dark period. It has been demonstrated that this excessive cracking of rubber is caused by atmospheric ozone formed in the photochemical smog formation process.
This type of smog can be invisible or it can appear as a whitish haze. This is verified by the fact that in Germany, emission of cement dusts decreased to one third while the production of cement tripled in the last years.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.